Analysis of metal material structures and strengthening methods
The performance of metallic materials is closely tied to their internal structure, which significantly influences their behavior under external stimuli such as mechanical stress, temperature variations, and environmental conditions. Understanding the structural characteristics of metals is essential for optimizing their versatile applications. As strengthening methods are an important means to improve the strength and durability of metal materials, we aim to analyze the various ways we treat our metal materials through the use of solid solution strengthening, work hardening and diffusion enhancement to develop high-performance metal materials.
How metal materials are organized:
Grain structure. Metals are composed of many grains, which are tiny, individual crystals that make up the microstructure of a solid metal. These grains are formed as metal solidifies from its molten state, with each grain having a specific orientation and arrangement of atoms. The size, shape and orientation of the grains have a significant impact on the properties of the metal. Generally, the finer the grains, the higher the strength and toughness of the metal. Grain refinement increases the area of grain boundaries (where these crystalline sheets meet), which hinder the movement of dislocations, making it more difficult for the metal to bend and deform, to improve its strength. Fine grains are also beneficial to improving the toughness of metals, as more energy is consumed when cracks propagate in these fine grains.
Phase composition. Metallic materials can contain multiple phases, such as solid solutions and intermetallic compounds, each contributing differently to the metal’s properties. A solid solution is a single, uniform phase formed when one compound (the solute) dissolves into another (the solvent) at the atomic level. Depending on how the atoms fit into the metal’s crystal structure—either by substituting out other atoms or fitting into the spaces between them—it can lead to solid solution strengthening. The added atoms create distortions in the lattice that hinder dislocation movement, which therefore increases the metal’s strength and hardness.
On the other hand, intermetallic compounds are distinct phases with specific stoichiometric ratios and often complex crystal structures. These compounds typically have high hardness and melting points. When they are finely and evenly dispersed within the metal matrix, they can significantly enhance the metal’s wear resistance, thermal stability, and overall durability. This combination of phases allows us to tailor the microstructure and performance of metallic materials for various applications.
Organizational morphology. The microstructure of metal materials can take various forms, including but not limited to equiaxed grains, columnar grains, and fibrous structures. Each of these have distinct characteristics that influence mechanical properties and performance. Understanding and controlling these structural morphologies is crucial to tailor the mechanical performance of metals for specific applications.
● Equiaxed grains are roughly equal in size in all directions and typically form during slow, uniform cooling or recrystallization. This structure is generally isotropic, as it has similar properties in all directions, and it offers a good balance of strength, ductility, and toughness. Equiaxed structures are often desirable in components that experience multi-directional stresses.
● Columnar grains are elongated crystals that grow in a preferred direction, usually during directional solidification, such as in casting or welding. These anisotropic grains provide higher strength and better mechanical properties along the grain growth direction, but their performance can be significantly weaker in the perpendicular direction. This can be a concern in applications where uniform strength in all directions is required.
● Fibrous structures form during plastic deformation processes like rolling, forging, or extrusion. In these cases, grains and inclusions become stretched and aligned along the working direction. This alignment results in high strength and improved toughness along the processing direction, but reduced strength also perpendicular to it. While this anisotropy can be beneficial in some structural applications, it may also pose limitations when loads are applied in other directions.
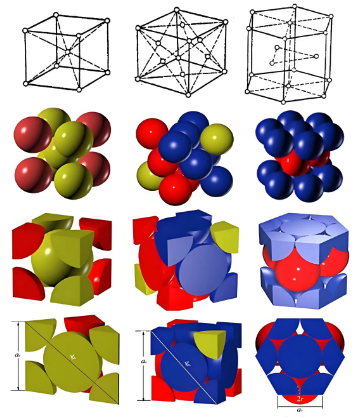
Figure 1. Various crystalline structures that can be observed in metal grains: body-centered cubic (left), face-centered cubic (middle), and hexagonal close packed (right).
Strengthening methods of metal materials:
Solid solution strengthening -
Principle. Adding solute atoms to the metal matrix can form a solid solution, further distorting the crystal lattice. This hinders the movement of dislocations in the crystal lattice and improves the strength and hardness of the metal.
Example. Adding manganese, nickel, chromium, and other metal elements to steel can form an alloy solid solution and improve the strength and toughness of the steel.
Work hardening -
Principle. During the plastic deformation process of metal, dislocation density of the metal grains increases and the interaction between dislocations strengthens. This makes it difficult for dislocations to move, thereby increasing the strength and hardness of the metal.
Example. Cold-drawn steel wire, cold-rolled steel plates, etc. are deformed through cold working, which greatly increases their strength; however, their plasticity and toughness will decrease.
Diffusion enhancement -
Principle. Fine dispersed second phase particles, such as carbides, nitrides, oxides, etc., are introduced into the metal matrix. These particles can hinder the movement of dislocations which improves the strength and hardness of the metal.
Example. Adding dispersed aluminum oxide particles to aluminum alloy can significantly improve the strength and heat resistance of aluminum alloy.

